Related Articles
CAR 7 – ASAIO Journal 2020
First in Human Experience with the Second Heart Assist Device L. W. Miller 1 , A. Ebner 2 , H. Leonhardt 3 , A. Richardson 4 , M. J. Cunningham 5 ; 1 Leslie W. Miller, MD, Dunedin, FL, 2 Universidad Nacional de Asuncion, Asuncion, Paraguay, 3 Second Heart Assist, Corona Del Mar, CA, 4 Second Heart Assist, Thousand Oaks, CA, 5 University of Southern California, Los Angeles, CA.
Study:
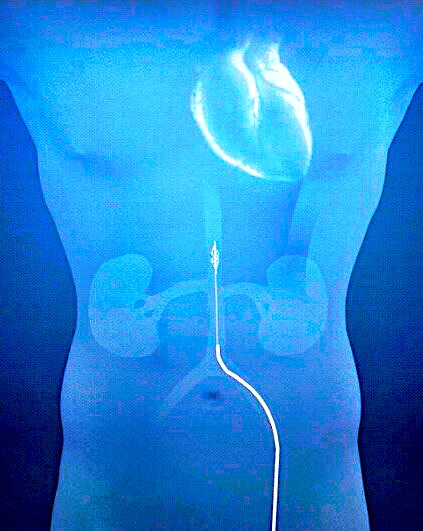
The Second Heart Assist Device is an impeller driven percutaneous temporary mechanical circulatory support device placed in the descending aorta that provides augmented pulsatile flow to the kidney and circulation. This First in Human study was designed to demonstrate the safety and feasibility of the device for support of patients undergoing high-risk percutaneous coronary intervention at a single center, Sanatorio Italiano, in Asuncion, Paraguay.
Methods: Four patients with reduced ejection fraction (30-40%) and complex coronary anatomy, underwent support with the Second Heart Assist device during elective PCI performed by radial approach.
Results: All patients had successful insertion of the SHA device via femoral access in less than two minutes. The pump was increased in speed to achieve a minimum of 10mmHg gradient across the pump, which averaged 8,500 RPMs (7,000-10,000). The duration of support was one hour in all patients. All patients had PCI of two vessels without complication or hemodynamic compromise. The device was removed percutaneously in all patients, and no patient experienced a serious Adverse Event. There was a four-fold average increase in urine output from baseline to end of support (mean 25 to 92.5 ml/min, and no increase in creatinine at discharge. (GFR data)
TCT THT March 2022
Totally Wireless and Waterproof Mechanical Circulatory Support (MCS) for Chronic Ambulatory Advanced Heart Failure (HF)
Leslie Miller, MD, Alex Richardson, Brett Burton, PhD, and Mark Cunningham, MD
Despite advances in medical management, HF is typically a progressive disease and of the 3 million patients with the reduced EF form in the US, 900,000 suffer with the advanced form, which is associated with multiple hospitalizations, high readmission rates with progressive decrease in survival with each subsequent admission, and poor quality of life. As such, advanced HF has been a target population for MCS for several decades. However, despite technological advances with large durable VADs, the high rates of serious adverse events including stroke, GI bleeding1, and driveline infection have limited their use in this population. The solution will require totally wireless power in a device designed to eliminate these complications. Previous attempts at wireless power have used Transcutaneous Energy Transfer(TET) that required surgical placement of a large inductive coil to receive external power from a large power source applied to the skin directly over the coil2, but this approach failed, and no solution has been pursued for over 25 years.
The Second Heart Assist device is a 13.5 French impeller-driven pump mounted on a driveshaft placed inside a stent cage delivered percutaneously into the descending aorta only 10 cm above the renal arteries, thereby eliminating the risk of stroke. It is the most efficient pump in the field, as it operates at only 7,500 RPMs and provides direct benefit to both the heart and kidneys. It improves cardiac output and reduces cardiac filling pressures due primarily to the afterload reduction created by activation of the impeller blades which pulls blood down through the pump, which can generate up to an additional 2.5 liters of augmented pulsatile flow over native cardiac output to the kidneys and the rest of the body. The increase in renal blood flow of up to 50% over baseline can offset the intra-renal vasoconstriction associated with low cardiac output in HF, leading to increased urine output, improved kidney function, and faster decongestion. The catheter-based first generation device can be inserted and the stent and impeller blades fully deployed in less than 2 minutes3. It is designed for 24 hours of support for patients admitted with ADHF who develop significant diuretic resistance. At the end of this support, the control handle can collapse the blades and stent for easy device removal of the stent and driveshaft.
The second-generation SHA Freedom device provides totally wireless power and eliminates the driveline without the need for a large sub-Q inductive coil, as the coil has been markedly miniaturized to fit on the distal tip of the catheter, along with the motor and battery. The stent and pump can be inserted and the driveshaft detached by demagnetizing a ball at the end of the driveshaft, leaving the device in close proximity to the kidneys(Figure 1). The power source is the size of a cell phone and is housed in a pocket inside a light-weight vest worn around the chest that has been made totally waterproof. The receiver coil can store enough power for the patient to electively remove the vest and take a shower with no attachments, as It is not providing life support, and allows patients to go in any body of water up to 30 meters, which is a first in the field, and a big advance in patient QOL. The risk of thrombosis is minimized by use of anti-coagulation and anti-vibrational technology. The wireless device can provide 72 hours of uninterrupted power, and is easily rechargeable, Blue-Tooth enabled, and also programmable to operate at as low as 2-3,000 RPMs as a partial support pump that can meet the individual patient support needs. It is intended for a wide range of chronic support duration from weeks to months or longer, such as during the vulnerable period after a HF hospitalization, as well as pre-transplant support, or in patients with HF and chronic kidney disease, or for ventricular recovery. Removal of the stent only requires the ball at the tip of a driveshaft to be re-magnetized to recapture the stent, and removed together, and another deployed if needed in the future.
In summary, a wirelessly powered MCS device has been developed that can provide significant support to both the heart and kidney of ambulatory patients with advanced HF and unprecedented improvements in patient QOL. The long-term goals of this device are to reduce hospitalizations, improve volume and diuretic management, and alter progression of native heart and kidney function, thereby also reducing cost of care.